M-910.04.950 [2024-02] Basic equipment in a brewery microbiology lab – Microbiological equipment
Application/Purpose
The method lists the equipment that is important in a brewery microbiology laboratory.
Scope of Application
Microbiology laboratories in the brewing and beverage industry and their suppliers.
Principle
Membrane filtration unit incl. vacuum pump and suction filter bottle
Membrane filtration is used for the microbiological analysis of liquids that can be filtered. Microorganisms are retained by a sterile filter membrane with a pore size of 0.45 µm or 0.2 µm. The membrane filters are then transferred to culture media for anaerobic or aerobic microorganisms and incubated.
Membrane filtration units can consist of individual filter funnels or filtration manifolds with several filter funnels.
|
Fig. 1: Membrane filtration unit consisting of stainless steel funnel, frit and Woulff bottle |
Fig. 2: Membrane filtration manifold for processing multiple samples |
 |
 |
The main differences lie in the type of funnel used. Typically, stainless steel funnels are used, which are sterilised before use using a Bunsen burner or autoclave. Other common options are reusable plastic funnels that can be sterilised in an autoclave or sterile disposable filter funnels that do not require sterilisation at all.
A membrane filter station can be placed in the main laboratory as long as the filter lids tightly seal the funnel. In the case of plastic funnels, suitable or tight-fitting lids are not always available, in which case filtration should be performed in a clean bench. This prevents any contaminated air from being sucked in.
Anaerobic jars/anaerobic systems
Anaerobic jars are used to culture anaerobic microorganisms. A prerequisite is that the containers have a gas-tight seal and do not allow oxygen to enter.
An anaerobic atmosphere can be generated in different ways:
-
The container is evacuated using a vacuum and then flushed with an oxygen-free gas mixture
-
The containers are filled with reaction packs, which remove the atmospheric oxygen in the container or generate a CO2 atmosphere. Different systems are available depending on the manufacturer.
-
The anaerobic jars, which are flushed with gas, are fitted with two valves on the lid, which are intended for flushing and evacuating. A pressure indicator on the lid measures the pressure in the jar.
Anaerobic jars without gas flushing are easier to set up, as they only need to be tightly sealed.
Fig. 3: Rectangular anaerobic jar for incubating anaerobic bacteria using oxygen-consuming chemicals
Anaerobic jars are available in metal, plastic and glass, with the glass and plastic containers having the advantage that the contents can be inspected from the outside without opening the lid, allowing you to identify an indicator change in the culture medium, for example.
Colony counters [1]
To count colonies on or in an agar plate, place transparent agar plates upside down on a flat, stable surface. It is important that the agar plate or its background is evenly illuminated without glare. It is also helpful to use a magnifying glass with 3-8x magnification or a stereomicroscope so that even very small colonies can still be seen. The counted colonies are usually marked with a coloured pencil on the underside of the Petri dish, or on the lid if using opaque culture media.
A more advanced method is to count colonies using a handheld colony counter. Here, the colony is counted using the tip of a pen, while the result is recorded on the LCD display.
A semi-automatic or automatic colony counter makes counting even more convenient and reliable.
Colony counters, although not all, offer three different ways of counting:
Counting by pressure:
This is the most common method, in which a coloured pen is used to lightly touch the (closed) Petri dish over the colony being counted. This marks the colony while pressing down slightly on the Petri dish; the pressure is transferred to a highly sensitive pressure plate, which triggers the counting pulse.
Counting based on electrical conductivity:
The Petri dish is open during the counting process. When a needle-shaped electrode is inserted into a colony, a current flows through the conductive culture medium to a counter electrode inserted into the agar at the edge of the Petri dish and this triggers the counting process. The method is unsuitable for (potentially) pathogenic microorganisms and is not recommended due to the risk of contamination.
Manual counting:
Counting in the conventional way by manually actuating a pressure switch in the appliance.
Cell counting using counting chambers [1]
The most common method for determining the total cell count in relatively high cell concentrations, e.g. pure yeast, is direct microscopic counting of the cells distributed in a counting chamber. The method is quick and requires little equipment.
The counting chamber method requires a relatively high cell concentration (> 107 cells/ml). In addition, the cells must be homogeneously distributed, immobile or immobilised and not be too small so that they can still be reliably identified under the microscope.
The counting chamber is a thick, flat-ground glass plate the size of a microscope slide, in the centre of which there are three parallel ridges ground crosswise, divided and delimited by grooves (Fig. 5).

Fig. 4: Thoma counting chamber
Source: https://de.wikipedia.org/wiki/Z%C3%A4hlkammer#/media/Datei:Neubauer_improved_counting_chamber.jpg (accessed on 17.11.2024)
Licence: https://commons.wikimedia.org/wiki/File:Neubauer_improved_counting_chamber.jpg?uselang=de#Lizenz (accessed on 17.11.2024)
The surface of the wider central ridge is ground to a precisely defined depth, slightly deeper than that of the two side ridges. A flat-ground, not-too-thin cover glass placed over the three ridges will rest only on the side ridges to create a hollow ("chamber") of a certain depth above the centre ridge.
The centre ridge is engraved with two square line grids separated by a transverse groove. The grid squares have a defined edge length and therefore a defined area, which means that the space above the square has a precisely defined volume at a known height. If this space is filled with a microorganism suspension and the cells contained in it are counted under the microscope, the number of cells per millilitre can be calculated.
Counting chambers with a depth of 0.02 mm are generally used for counting bacteria, while chambers with a depth of 0.1 mm are used for counting larger microorganisms such as yeasts.
The counting area consists of 16 large squares, which are bordered or separated by rows of smaller squares with an additional central limiting line. There are different versions of the Thoma chamber; the widely used old Neubauer version is shown here, consisting of 16 large squares. The improved Neubauer version consists of 25 large squares.
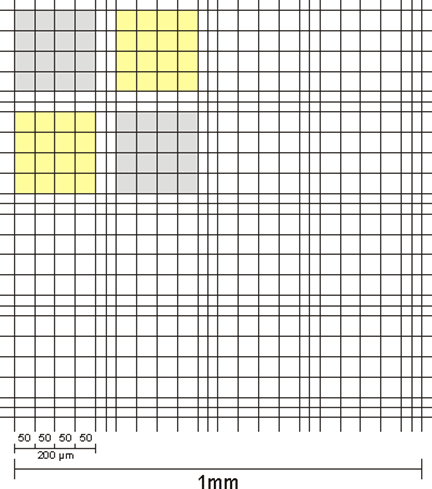
Fig. 5: General view of the counting area of a Thoma chamber (old Neubauer version)
Source: https://de.wikipedia.org/wiki/Z%C3%A4hlkammer#Z%C3%A4hlkammer_nach_Neubauer (accessed on 19.10.2024)
Licence: https://commons.wikimedia.org/wiki/File:Neubauer_classic_center_square.gif?uselang=de#Lizenz (accessed on 19.10.2024)
The cell count per ml of undiluted microorganism suspension is calculated using the following formula:
\(\text{cell count/ml}=\frac{\text{Total number of counted cells } \times \text{ dilution factor } \times 4 \times10^8}{\text{Number of small squares counted }\times\text{ chamber depth in µm}}\)